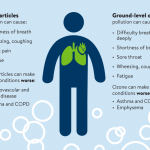
The clean room technology developed from the 1950s to the 1960s is a comprehensive technology gradually formed to cater to the development of modern electronics, aerospace, and precision machinery. Later, it was found that other modern industries, pharmaceuticals, and hospitals that treat leukemia, burns, or perform major operations all have high requirements for the cleanliness of the indoor environment. At present, it can be said that the level of air cleanliness and air purification technology has directly affected the improvement and development of modern science and technology. Air cleanliness, as far as its basic control objects are concerned, can also be called aerosol science. It is this particulate matter that becomes an aerosol that the clean room needs to control. Around the same time as the development of clean room technology, the contamination of indoor air was discovered. Indoor air pollution is due to the extensive use of air conditioners and the airtightness of modern buildings is getting better and better. Closed environments with poor air circulation create a variety of indoor air pollution problems. Some people classify indoor air pollution into four kinds of pollution, namely: physical pollution, chemical pollution, biological pollution and radioactive pollution. Later, it was discovered that aerosol pollution presents the above four types of pollution. Aerosols are granular, and its size determines that it can enter our respiratory system with the airflow, and the fine particles in it may also enter the human circulatory system and affect human health. Aerosol will become the carrier of many chemical pollutants, metal elements and their compounds and radioactive substances, sulfates, nitrates, silicates and many complex organic compounds will cause damage to the human body through aerosol carriers. It must be pointed out that various pathogenic microorganisms (bacteria, viruses and fungi) also exist in the form of aerosols in the indoor air and pose a threat to human health.
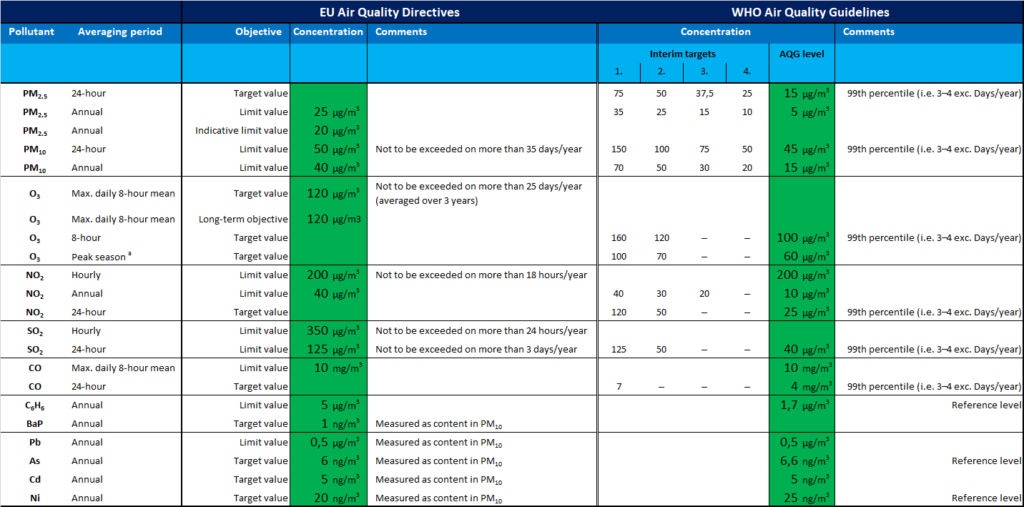
In 1975, the European Union adopted its first regulation on air pollution prevention and control – gasoline sulfur content directive. It was not until the mid-1980s that the European Union gradually strengthened air pollution legislation due to the aggravation of European air pollution, acid rain, ozone layer depletion, and global warming. The EU has passed nearly 20 regulations and directives on gas and dust emissions, nine conventions, decisions and directives on the protection of the ozone layer, and formulated a number of regulations on the cooperation of member states in air pollution prevention and control, forming a fairly complete system. legal system. Different experts describe particles in terms of shape, size and chemical composition. Toxicologists refer to aerosols as ultrafine, fine, or coarse substances. Regulators, as well as meteorologists, often call them particulate matter —PM2.5 or PM 10, depending on their size. In some engineering fields, they are called nanoparticles. The media often uses everyday terms that imply the source of aerosols, such as smoke, ash, and soot.
Climatologists typically use another set of labels related to chemical composition. The main aerosol groups include sulfates, organic carbon, black carbon, nitrates, mineral dust and sea salt. In practice, many of these terms are imperfect because aerosols often aggregate together to form complex mixtures. For example, it is common for black carbon particles in soot or smoke to mix with nitrates and sulfates, or to coat dust surfaces, creating mixed particles. Most aerosols – about 90% by mass – are of natural origin. Volcanoes, for example, spew huge plumes of ash into the air along with sulfur dioxide and other gases, producing sulfates. Forest fires send some of the burned organic carbon aloft. Gases produced by certain plants react with other substances in the air to produce aerosols, such as the “smog” of the Great Smoky Mountains in the United States. Likewise, in the ocean, certain types of microalgae produce a sulfurous acid gas called dimethyl sulfide, which can be converted to sulfate in the atmosphere. Sea salt and dust are two of the most abundant aerosols, as dust storms blow small pieces of mineral dust from deserts into the atmosphere, and wind-driven spray from ocean waves throws sea salt high into the air. Both tend to be particles that are larger than man-made particles. The remaining 10 percent of aerosols are considered anthropogenic, or anthropogenic, and come from a variety of sources. Although not as abundant as natural forms, anthropogenic aerosols can dominate the air downwind of cities and industrial areas. Combustion of fossil fuels produces large amounts of sulfur dioxide, which reacts with water vapor and other gases in the atmosphere to produce sulfate aerosols. Biomass burning, a common method for clearing land and consuming farm waste, produces smog composed mainly of organic and black carbon. Automobiles, incinerators, smelters and power plants are prolific producers of sulfates, nitrates, black carbon and other particulates. Deforestation, overgrazing, drought and over-irrigation alter the surface and increase the rate at which dust aerosols enter the atmosphere. Even indoors, cigarettes, stoves, fireplaces, and candles are sources of aerosols.